
The horizontal part of the graph shows that the salol has a sharp melting point, so it is pure. The temperature stays the same while a pure substance changes state The graph shows the cooling curve for a sample of a compound called salol. This difference is most easily seen when the temperature of a hot liquid is measured as it cools and freezes. Pure substances have a sharp melting point but mixtures melt over a range of temperatures. The substances in the mixture can be separated.Ĭannot be separated but can be obtained by using chemical reactions.ĭistinguishing between pure substances and mixtures Properties are different from those of the elements it contains. Keeps the properties of the substances involved. The hydrogen and oxygen have joined together to form the new substance water. The hydrogen and oxygen are not joined together. Definition History Chemical elements Chemical compounds Substances versus mixtures Chemicals versus chemical substances Naming and indexing See also. This ratio is shown in the chemical formula of the compound - H 2 O. Variable composition - the relative amounts of the two gases can be changed.Ĭonstant composition - water always contains the same ratio of hydrogen to oxygen.
In this table, the column 'Mixture' refers to the gasses hydrogen and oxygen, and the column named 'Compound' refers to water. There are important differences between the properties of a mixture and a compound. Water is a compound of hydrogen and oxygen. Apart from this, various components are mixed thoroughly with each. Together, as a mixture, hydrogen and oxygen can react and form water. This is because its composition in terms of its components is not fixed. The components of a mixture can be separated without chemical reactions Mixtures and compounds

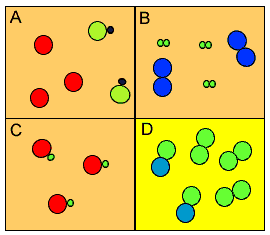
a mixture consists of two or more different substances, not chemically joined together Then describe its composition as elements only, compounds only, or elements and.concrete Answer a) Methane ( C H 4) is a compound because it is made up of two different elements which are joined by chemical bond. a pure substance consists only of one element or one compound Identify each of the following as an element, a compound, a homogeneous mixture, or a heterogeneous mixture.However, the juice is not pure in the chemical sense because it contains different substances mixed together. The label means that the contents are just orange juice, with no other substances added. For example, shops sell cartons labelled as 'pure' orange juice. The word 'pure' is used in chemistry in a different way from its everyday meaning. What we wouldn't describe him as a mixture, since it is just that those few atoms bound together.Pure substances and mixtures The meaning of pure They're with the combination of multiple elements, water each to home, which are Mars saying without just that same formula before their compounds because they're more couple getting the element. This one existence, it's um, a mixture of different dressings in order to cross street on water and ammonia or to meeting ones are both compounds. Oh, gee, isn't that have heterogeneous? Jumping down to E mustard once again is a hatter genius mixture. Since there are some confusion to be seen without, it's gonna be the same percent off oxygen versus nature gen X address. Identify each of the following as element, compound, homogeneous mixture, or heterogeneous mixture. You have different cell types, different compounds and is a heterogeneous mixture, not a home watching.

For instance, there are so many different particles making a blood. Next I'm going to blood, so we know for sure blood is that element or compound. It's not a combined combined with anything else. C Isn't element confined into me on a, um, periodic table. We have to decide what each of these are that your element compound? Oh, hetero genius mixture or homogeneous gonna start off with the easiest one.


 0 kommentar(er)
0 kommentar(er)
